drug dilution guidelines
Name of person who made the dilution. This guideline was prepared to facilitate the healthcare staffs in.
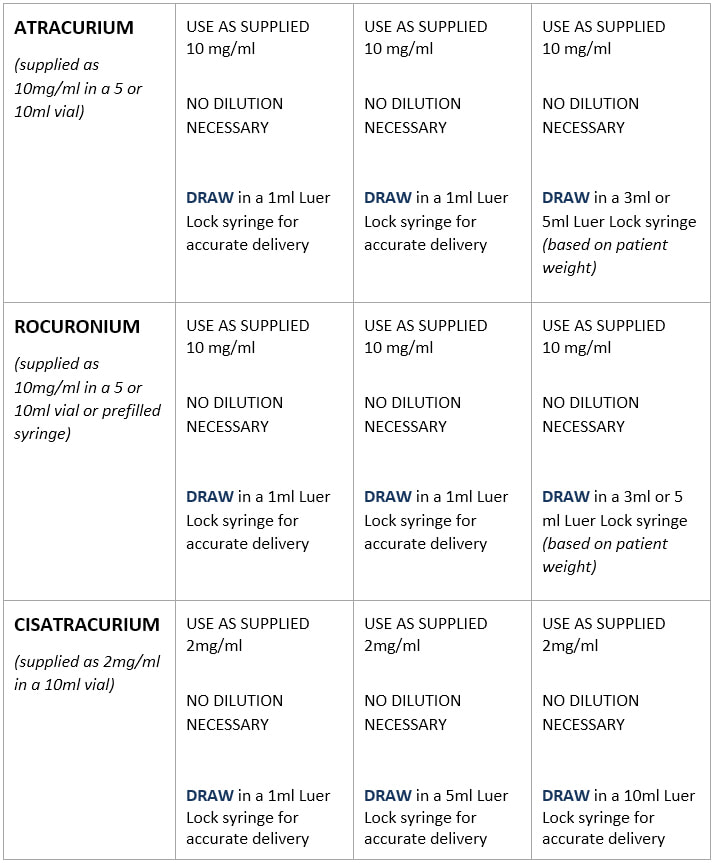
Standard Drug Dilutions In The Pediatric Or Pediatric Anesthesia Digital Handbook
The policies described in the final and revised draft guidances are intended to minimize public health risks that repackaging drug products and mixing diluting or repackaging biological.

. Dilution Guideline for Injectable Drugs serves as a general reference for the healthcare professionals in the Ministry of Health on how to prepare dilution of certain injectable drugs before administering to the patient. As a pharmacy student its vital then that you have a. January 20 2021 _____ Drug Dilution and Storage Guidelines Most of the drugs used for laboratory animals ie mice rats need to be.
Each drug is limited to one or two recommended concentrations that will serve the needs of most patients in the care settings where the drug is most commonly used. IV 5-20mg bolus injected over 2min max 200mg Infusion dose. Dilution of IV push medications should be done only when either required by the manufacturer based on peer-reviewed evidence or by institutional guidelines.
Drug Dilution and Storage Guidelines DATE. Dilutions are an important topic in pharmacy calculations. So if there is 1g per 100mls there is also 1000mg per 100mls.
No respondents described a dilution process that would result in a specific concentration eg add 4 mL of diluent to 1 mL of drug to equal xx mg5 mL. 5ml of lidocaine will have 5 x 10mg 50mg in a 5ml vial. Dose of 100 mg and more.
Establish Dilution Guidelines for Injectable Drugs topromote safe medication use. 14 April 2021 - 319pm. Intravenous admixtures preparation and infusion guidelines.
X Dilute to 2 InfusionMAX concentration of 2 mgmL over 1 hr Dosing. Antimicrobial Antifungal and Antiviral Drugs in Pediatrics University Malaya Medical Center -. Do Not Administer 1 Revised.
If we divide this down there will be 10mg in 1ml. It may be demonstrated directly on the drug substance by dilution of a standard stock solution andor separate weighings of synthetic mixtures of the drug product components using the. No other dilution required Dose of 99 mg and less.
Labetalol Preparation. Drug Dilution. As suggested by the variability of.
Up to 24 cash back Dilute the 1 g vial with 95 mL of SWI to obtain a final concentration of 100 mgmL. The 8020 rule the. 30mgkg X 1 MAX 1500mg For specific indications.
User should utilize appropriate drug reference books for complete administration instructions and drug information. This reference contains standard dilutions including IV admixture drug concentration infusion volumes and. 5-mL and 10-mL normal saline NS.
UMMC Pediatrics Dilution Protocol for Antimicrobials Antifungals and Antivirals. All diluted drugs must be labeled with the following information. Expiration date which is thirty 30 days after the.
Use of normal saline for dilution of drugs is a common practice especially for paediatric patients and for inotropic medications. In terms of dilution 1 is the same as a 1100 solution dilution. Dilution reconstitution or the safe rate of administration of IV push medications Risks Associated with Drug Labeling Packaging and Nomenclature IV medications that are prepared in empty.
A 1 solution literally means there is 1gram per 100mls. Therefore correct dilution and administration of injectable medications are crucial to ensure patients safety. Dose 20-160mghr Dilute in D5 or DS Method 1.
However this procedure is considered. Moreover errors in intravenously administered medications may have serious consequences and incident. Infusions Guidelines v40 General Critical Care July 2021 7 n-ACETYL CYSTEINE INFUSION Review January 2022 Date Drug Dose Route ddmmyy N-ACETYL.
25mg 5ml Bolus dose. With the dilution of a medicine drug concentration changes. 5-10 mgkgday as q 24 h MAX 500 mg Single dose regimen.

Dilution Chart Magnet Plant Therapy
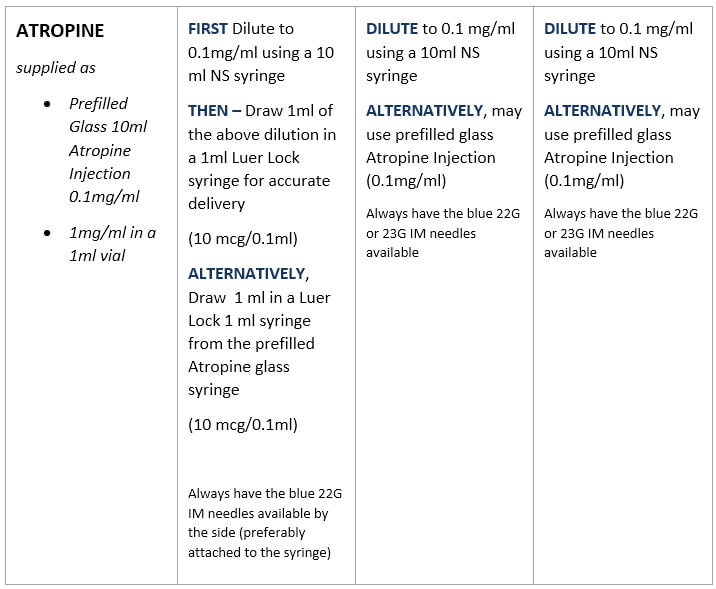
Standard Drug Dilutions In The Pediatric Or Pediatric Anesthesia Digital Handbook

Serial Vs Direct Dilution Time To Apply New Thinking To Ic50 Determination And Dose Response Analysis Drug Discovery World Ddw
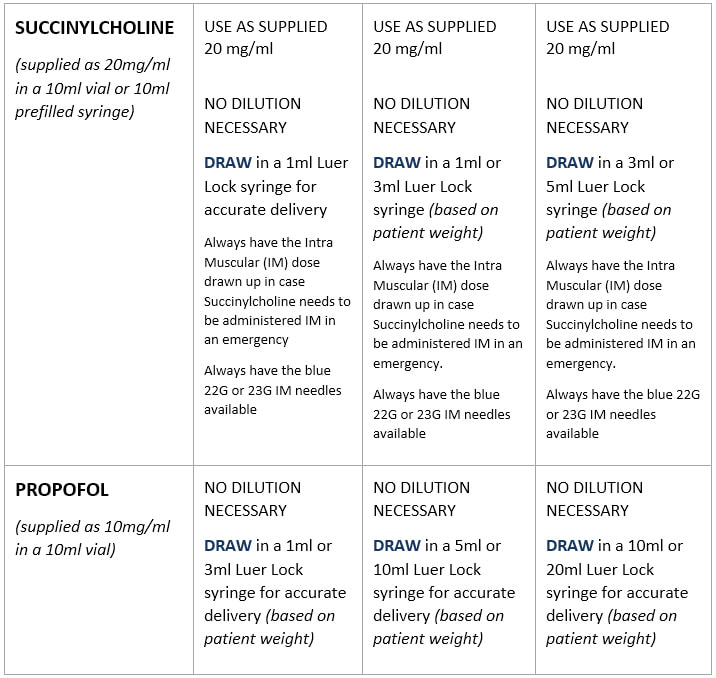
Standard Drug Dilutions In The Pediatric Or Pediatric Anesthesia Digital Handbook
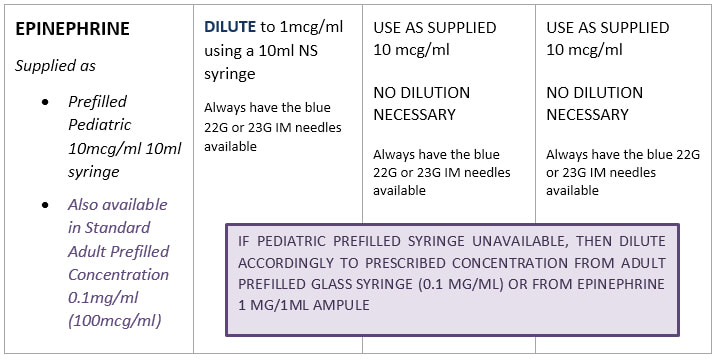
Standard Drug Dilutions In The Pediatric Or Pediatric Anesthesia Digital Handbook
Some Iv Medications Are Diluted Unnecessarily In Patient Care Areas Creating Undue Risk Institute For Safe Medication Practices
Wall Chart Drugs Given By Iv Push Or Rapid Administration In Adults Institute For Safe Medication Practices
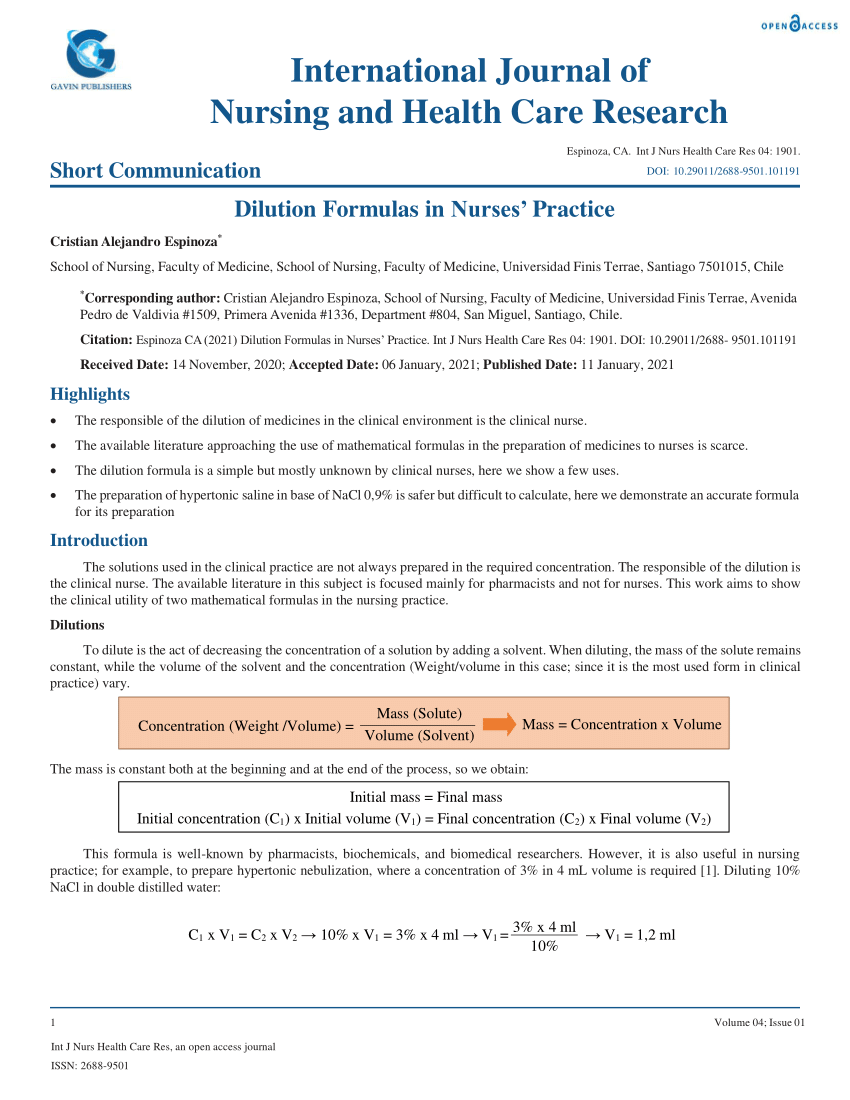
Pdf Dilution Formulas In Nurses Practice
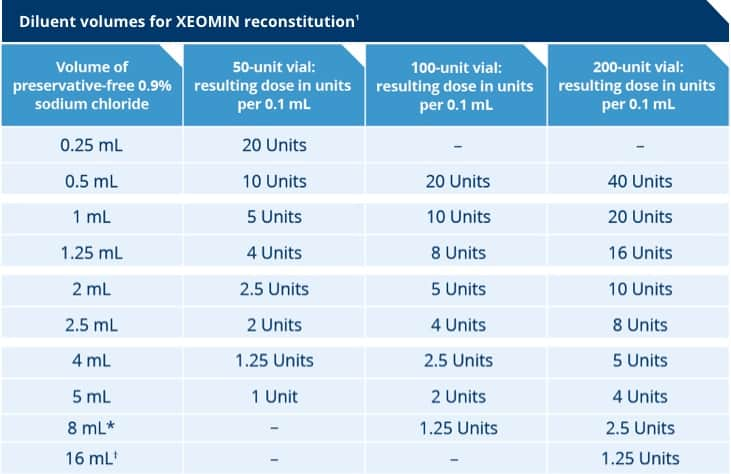
Reconstitution Dilution And Storage Xeomin

Antimicrobial Susceptibility Testing Of Mycobacterium Tuberculosis Complex Isolates The Eucast Broth Microdilution Reference Method For Mic Determination Clinical Microbiology And Infection

Serial Vs Direct Dilution Time To Apply New Thinking To Ic50 Determination And Dose Response Analysis Drug Discovery World Ddw

Serial Dilution Methods Calaculations Youtube






0 Response to "drug dilution guidelines"
Post a Comment